
KESHAVARZI Sepiedeh
- The Keshavarzi lab, University of Cambridge, Cambridge, United Kingdom
- Electrophysiology, Interneurons, Neuronal assembly, Synaptic network, Systems/Circuit Neuroscience
- recommender
Recommendation: 1
Reviews: 0
Recommendation: 1
Functional correlates of immediate early gene expression in mouse visual cortex
Bringing together immediate early genes and sensorimotor response properties in V1
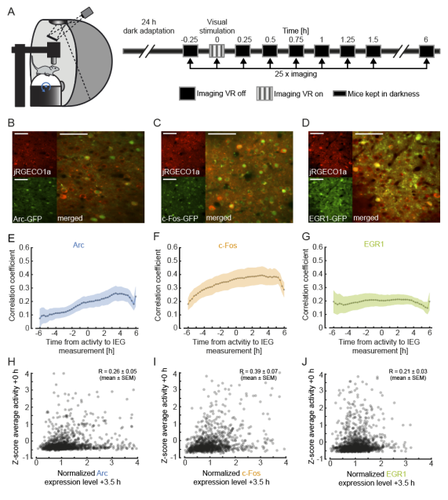
Recommended by Julia Jade Harris and Sepiedeh Keshavarzi based on reviews by Balázs Hangya and 2 anonymous reviewersThe primary visual cortex (V1) does not just process vision: it also integrates self-generated motion signals (Niell, Stryker 2010; Keller et al. 2012; Saleem et al. 2013; Vélez-Fort et al. 2018; Meyer et al. 2018), enabling us to match our actions to the world we see. We know that the development of visuomotor representation in V1 depends on experience (Attinger et al. 2017; Widmer et al. 2022), but how exactly does each neuron acquire the right balance of visual and motor input? And how do some neurons become more responsive to visual or motor signals? Mahringer et al. (Mahringer et al. 2022) suspected that the answers may lie in experience-specific plasticity mechanisms.
To investigate this, the authors measured the expression of immediate early genes (IEGs) as indicators of both past neural activity and future plasticity. They examined three IEGs previously implicated in visual cortical plasticity: c-fos, egr1 and Arc (Yamada et al. 1999; Wang et al. 2006; Xie et al. 2014). In three separate transgenic mouse lines, GFP expression was driven by these IEGs, and a red variant of a genetically encoded calcium indicator allowed for simultaneous measurement of neuronal activity. Initial characterisation of IEG expression and calcium fluorescence revealed that IEG levels were only weakly (positively) correlated with visually-evoked neural activity.
But what about the relationship between IEG expression and first visual or visuomotor experience? In dark-reared mice, first visual and visuomotor experiences led to differential IEG expression: Arc expression increased after first visual and visuomotor experiences; EGR1 expression decreased after first visuomotor experience; and c-Fos expression remained largely unchanged. Neural activity levels could not account for these changes, suggesting that different sensory experiences can selectively recruit different IEG expression patterns, perhaps according to input pathway.
Further analysis of those neurons with the highest levels of IEG expression revealed that different IEGs were associated with different functional response properties. High Arc-expressing neurons developed above-average visual and below-average motor responses, while high EGR1-expressing neurons developed above-average motor responses. These results suggest that during experience-dependent wiring, Arc expression drives plasticity favouring bottom-up visual input, while EGR1 expression drives plasticity favouring top-down motor input. Interestingly, while Arc-expressing neurons appear to end up with little-to-no motor input, EGR1-expressing neurons appear to enjoy both visual and motor input, enabling them to display above-average visuomotor mismatch responses.
Overall, this work makes two important advances. First, it suggests that IEG expression may be more closely linked to specific forms of plasticity than general levels of neural activity. Second, it reveals a mechanism by which visual cortical neurons can acquire specific functional properties by selectively upregulating bottom-up or top-down inputs in response to particular sensory experiences.
As an additional note, we would like to highlight a vigorous technical discussion that this manuscript triggered: unconventionally, the authors chose not to apply a neuropil correction procedure to their calcium imaging data. This decision split opinion, amongst both reviewers and recommenders. We have come to the view that the findings are nevertheless of interest for the community and are pleased to point readers towards the publicly available reviews and authors’ responses.
Attinger A, Wang B, Keller GB (2017) Visuomotor Coupling Shapes the Functional Development of Mouse Visual Cortex. Cell, 169, 1291-1302.e14. https://doi.org/10.1016/j.cell.2017.05.023
Keller GB, Bonhoeffer T, Hübener M (2012) Sensorimotor mismatch signals in primary visual cortex of the behaving mouse. Neuron, 74, 809–815. https://doi.org/10.1016/j.neuron.2012.03.040
Mahringer D, Zmarz P, Okuno H, Bito H, Keller GB (2022) Functional correlates of immediate early gene expression in mouse visual cortex. bioRxiv, 2020.11.12.379909, ver. 4 peer-reviewed and recommended by Peer community in Neuroscience. https://doi.org/10.1101/2020.11.12.379909
Meyer AF, Poort J, O’Keefe J, Sahani M, Linden JF (2018) A Head-Mounted Camera System Integrates Detailed Behavioral Monitoring with Multichannel Electrophysiology in Freely Moving Mice. Neuron, 100, 46-60.e7. https://doi.org/10.1016/j.neuron.2018.09.020
Niell CM, Stryker MP (2010) Modulation of Visual Responses by Behavioral State in Mouse Visual Cortex. Neuron, 65, 472–479. https://doi.org/10.1016/j.neuron.2010.01.033
Saleem AB, Ayaz A, Jeffery KJ, Harris KD, Carandini M (2013) Integration of visual motion and locomotion in mouse visual cortex. Nature Neuroscience, 16, 1864–1869. https://doi.org/10.1038/nn.3567
Vélez-Fort M, Bracey EF, Keshavarzi S, Rousseau CV, Cossell L, Lenzi SC, Strom M, Margrie TW (2018) A Circuit for Integration of Head- and Visual-Motion Signals in Layer 6 of Mouse Primary Visual Cortex. Neuron, 98, 179-191.e6. https://doi.org/10.1016/j.neuron.2018.02.023
Wang KH, Majewska A, Schummers J, Farley B, Hu C, Sur M, Tonegawa S (2006) In Vivo Two-Photon Imaging Reveals a Role of Arc in Enhancing Orientation Specificity in Visual Cortex. Cell, 126, 389–402. https://doi.org/10.1016/j.cell.2006.06.038
Widmer FC, O’Toole SM, Keller GB (2022) NMDA receptors in visual cortex are necessary for normal visuomotor integration and skill learning. eLife, 11, e71476. https://doi.org/10.7554/eLife.71476
Xie H, Liu Y, Zhu Y, Ding X, Yang Y, Guan J-S (2014) In vivo imaging of immediate early gene expression reveals layer-specific memory traces in the mammalian brain. Proceedings of the National Academy of Sciences, 111, 2788–2793. https://doi.org/10.1073/pnas.1316808111
Yamada Y, Hada Y, Imamura K, Mataga N, Watanabe Y, Yamamoto M (1999) Differential expression of immediate-early genes, c-fos and zif268, in the visual cortex of young rats: effects of a noradrenergic neurotoxin on their expression. Neuroscience, 92, 473–484. https://doi.org/10.1016/S0306-4522(99)00003-2