
RANCZ Ede
- Cortical Circuits Lab, INMED, INSERM, Marseille, France
- Electrophysiology, Methods development, Neuronal assembly, Rodent model system, Synaptic network, Systems/Circuit Neuroscience
- recommender
Recommendations: 0
Review: 1
Review: 1
The Switchmaze: an open-design device for measuring motivation and drive switching in mice
Novel automated training platform for studying flexible switching among natural motivated behaviors in mice
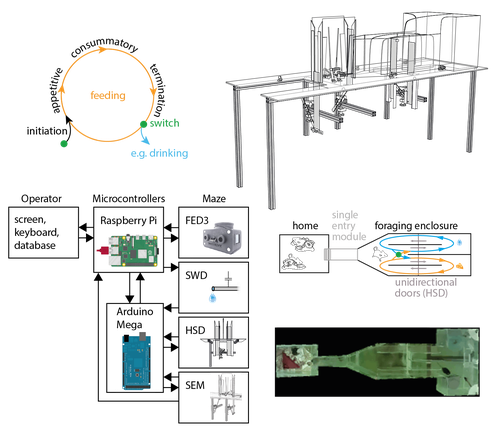
Recommended by Balázs Hangya based on reviews by Ede Rancz and Ewelina KnapskaAs our understanding of the building blocks of mammalian behavior improves, there is a shifting focus towards addressing the brain mechanisms of behavioral flexibility, strategy learning and behavioral switching (Banerjee et al., 2020; López-Yépez et al., 2021; Manzur et al., 2023). This requires novel behavioral paradigms and new tools: Hartmann and colleagues started filling this gap by presenting an open-source automated training system for studying motivational switching, which they coined the ‘Switchmaze’ (Hartmann et al., 2024).
Instead of training mice on specific tasks, Hartmann et al. chose to quantify switching between spontaneous motivated behaviors like feeding, drinking, and engaging in social interactions in a type of foraging task. These behaviors were spatially separated by a smart design using distinct compartments with unidirectional doors, allowing the counting of discrete cycles of food and water intake in unitary quantities.
Switching behavior was quantified by the ratio of shifting from one behavioral chamber to another (single probe entries) versus exploitation of a single chamber through multiple consecutive entries (continuous exploitation runs), termed ‘motivation switching rate’ (MSR). Interestingly, the measured MSR values were well within the distribution of randomized data in which the trial sequence was shuffled. The Authors suggest that this may be an adaptive strategy to decrease behavioral predictability and thus fool competitors and predators; however, determining the significance of this finding will require further testing. For instance, is a ‘more random mouse’ indeed more successful in a competitive setting where the total amounts of food and water are limited? Food deprivation increased, while re-feeding decreased switch rate, strengthening the arguments for the MSR being a strategically controlled parameter.
Hartmann et al. further demonstrated the utility of the Switchmaze by performing chemogenetic inhibition of prefrontal cortical neurons projecting to the hypothalamus, a pathway thought to be involved in controlling feeding behavior (Petrovich et al., 2005; Cole et al., 2020; Padilla-Coreano et al., 2022). Mice showed an increased MSR upon inhibition, now significantly different from randomized distributions. Further analysis revealed that the difference was driven by a selective reduction of food-to-food transitions, that is, a decreased tendency for repetitive feeding. Moreover, this was due to a decrease in the number but not the duration of food runs, suggesting a specific behavioral role of the prefrontal-hypothalamic pathway in promoting repetitive feeding.
In summary, Hartmann and colleagues showcased an affordable, open-source behavioral design and demonstrated its usefulness for quantifying flexible switching of natural behaviors. It is ideal for testing the effect of pharmacological and chemogenetic manipulations, but it can likely be combined with electrophysiology, fiber photometry or miniscope imaging, greatly broadening its potential. Therefore, the Switchmaze is a valuable member of the growing family of open source, automated rodent training tools (Puścian et al., 2016; Erskine et al., 2019; Qiao et al., 2019; Birtalan et al., 2020; Cano-Ferrer et al., 2024) that represent the logical next step for high-throughput, stress- and bias-free behavioral experimentation.
References
Banerjee A, Parente G, Teutsch J, Lewis C, Voigt FF, Helmchen F (2020) Value-guided remapping of sensory cortex by lateral orbitofrontal cortex. Nature 585:245–250 Available at: http://dx.doi.org/10.1038/s41586-020-2704-z.
Birtalan E, Bánhidi A, Sanders JI, Balázsfi D, Hangya B (2020) Efficient training of mice on the 5-choice serial reaction time task in an automated rodent training system. Sci Rep 10:22362 Available at: https://doi.org/10.1038/s41598-020-79290-2.
Cano-Ferrer X, Tran-Van-Minh A, Rancz E (2024) RPM: An open-source Rotation Platform for open- and closed-loop vestibular stimulation in head-fixed Mice. J Neurosci Methods 401:110002 Available at: https://doi.org/10.1016/j.jneumeth.2023.110002.
Cole S, Keefer SE, Anderson LC, Petrovich GD (2020) Medial Prefrontal Cortex Neural Plasticity, Orexin Receptor 1 Signaling, and Connectivity with the Lateral Hypothalamus Are Necessary in Cue-Potentiated Feeding. J Neurosci 40:1744–1755 Available at: https://www.jneurosci.org/lookup/doi/10.1523/JNEUROSCI.1803-19.2020.
Erskine A, Bus T, Herb JT, Schaefer AT (2019) AutonoMouse: High throughput operant conditioning reveals progressive impairment with graded olfactory bulb lesions Reisert J, ed. PLoS One 14:e0211571 Available at: https://dx.plos.org/10.1371/journal.pone.0211571.
Hartmann C, Mahajan A, Borges V, Razenberg L, Thönnes Y, Karnani MM (2024) The Switchmaze: an open-design device for measuring motivation and drive switching in mice. bioRxiv:1–17 Available at: https://doi.org/10.1101/2024.01.31.578188.
López-Yépez JS, Martin J, Hulme O, Kvitsiani D (2021) Choice history effects in mice and humans improve reward harvesting efficiency Palminteri S, ed. PLOS Comput Biol 17:e1009452 Available at: https://dx.plos.org/10.1371/journal.pcbi.1009452.
Manzur HE, Vlasov K, Jhong Y-J, Chen H-Y, Lin S-C (2023) The behavioral signature of stepwise learning strategy in male rats and its neural correlate in the basal forebrain. Nat Commun 14:4415 Available at: https://www.nature.com/articles/s41467-023-40145-9.
Padilla-Coreano N et al. (2022) Cortical ensembles orchestrate social competition through hypothalamic outputs. Nature 603:667–671 Available at: https://www.nature.com/articles/s41586-022-04507-5.
Petrovich GD, Holland PC, Gallagher M (2005) Amygdalar and Prefrontal Pathways to the Lateral Hypothalamus Are Activated by a Learned Cue That Stimulates Eating. J Neurosci 25:8295–8302 Available at: https://www.jneurosci.org/lookup/doi/10.1523/JNEUROSCI.2480-05.2005.
Puścian A, Łęski S, Kasprowicz G, Winiarski M, Borowska J, Nikolaev T, Boguszewski PM, Lipp H-P, Knapska E (2016) Eco-HAB as a fully automated and ecologically relevant assessment of social impairments in mouse models of autism. Elife 5:1–22 Available at: https://elifesciences.org/articles/19532.
Qiao M, Zhang T, Segalin C, Sam S, Perona P, Meister M (2019) Mouse Academy: high-throughput automated training and trial-by-trial behavioral analysis during learning. bioRxiv:467878 Available at: http://biorxiv.org/content/early/2019/02/13/467878.abstract.